Vaginal atrophy affects many of your patients
Vaginal atrophy is a common issue
Vaginal atrophy is a significant problem amongst women who have gone through the menopause. About half of all postmenopausal women experience discomfort due to vaginal atrophy.1
Vaginal atrophy is undertreated
50% of postmenopausal women with genitourinary symptoms have never used any therapy for this problem.2
Patients need your help to talk about vaginal atrophy
Women want accurate medical information about vaginal atrophy, but they are more likely to want the HCP to initiate the conversation.3


Vaginal atrophy is a common issue
Vaginal atrophy is a significant problem amongst women who have gone through the menopause. About half of all postmenopausal women experience discomfort due to vaginal atrophy.1
Vaginal atrophy is undertreated
50% of postmenopausal women with genitourinary symptoms have never used any therapy for this problem.2
Patients need your help to talk about vaginal atrophy
Women want accurate medical information about vaginal atrophy, but they are more likely to want the HCP to initiate the conversation.3
Symptoms
Vaginal atrophy has many symptoms which affect every part of her life4.

Vaginal dryness

Dyspareunia

Itching

Dysuria

Burning

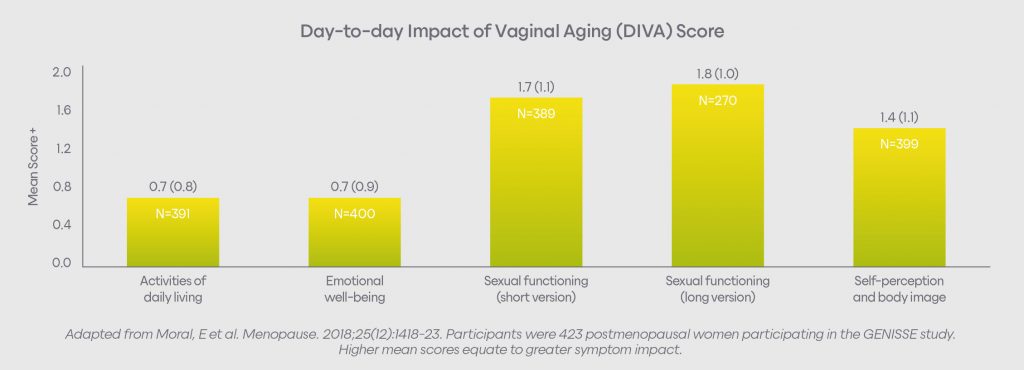
Vaginal symptoms impact women in many ways, including:5
Diagnosis

Women need prompt diagnosis and treatment
When you identify vaginal atrophy, it may favour early management and treatment.6
NICE guidelines suggest local treatment over systemic hormonal replacement therapy.7
They state that vaginal oestrogen should be offered to women to treat the symptoms of vaginal atrophy.7

When choosing a treatment for vaginal atrophy, the dose is important
The British Menopause Society WHC guideline advocates to use the lowest effective HRT dose for treating symptoms.8

Release her confidence from within.
To book a visit from a Consilient Healthcare BlisselⓇ trained healthcare manager, please click here to complete our DATA compliant form:

Ultra low dose estriol vaginal gel9,10
Prescribing and Adverse event reporting information for BlisselⓇ can be found here.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
1.Nappi RE. and Palacios S. Climacteric. 2014;17:3–9. 2. NAMS position statement. Menopause. 2020;27(9):976-92. 3. Krychman M, et al. J Sex Med 2017;14:425-433. 4. Palmaa F, et al. Maturitas. 2018;108:18–23. 5. Moral E, et al. Menopause. 2018;25(12):1418-23. 6. Cagnacci A, et al. Climacteric. 2019;22(1):85-89. 7. NICE. Menopause: diagnosis and management (NG23). Available from: https://www.nice.org.uk/guidance/ng23/chapter/recommendations. Accessed December 2022. 8. British Menopause Society Women’s Health Concern Fact Sheet: HRT Benefits and Risks; Nov 2017. 9. Cano A, et al. Menopause. 2012;19(10):1130-9.
10. BlisselⓇ 50 micrograms/g vaginal gel. Summary of product characteristics.
Date of preparation: March 2025 | UK-BLS-43b(2)



